
There is a handful of very primitive concepts in thermodynamics. See also: Atom Matter Statistical mechanics
QUIZLET SECOND LAW OF THERMODYNAMICS SIMPLE STATES THAT FULL
However, interpretations in terms of the statistical behavior of large assemblies of particles greatly enriches the understanding of the relations established by thermodynamics, and a full description of nature should use explanations that move effortlessly between the two modes of discourse. Classical thermodynamics consists of a collection of mathematical relations between observables, and as such is independent of any underlying model of matter (in terms, for instance, of atoms). The connection between phenomenological thermodynamics and the properties of the constituent particles of a system is established by statistical thermodynamics, also called statistical mechanics. Each law embodies a particular constraint on the properties of the world. The subject of thermodynamics is founded on four generalizations of experience, which are called the laws of thermodynamics. See also: Chemistry Energy Heat Newton's laws of motion Physics Refrigeration Refrigerator Temperature 1) and refrigerators (devices that use external sources of work to transfer heat from a hot system to cooler sinks), and for discussing the spontaneity of chemical reactions (their tendency to occur naturally) and the work that they can be used to generate. In practice, thermodynamics is useful for assessing the efficiencies of heat engines (devices that transform heat into work, see Fig. It differs from the dynamics of English physicist and mathematician Isaac Newton by taking into account the concept of temperature, which is outside the scope of classical mechanics. Thermodynamics is the science of the transformation of energy. Laws governing the transformation of energy.
As a consequence, as the temperature of a substance approaches absolute zero, the entropy will decrease to a steady minimum value. The third law of thermodynamics states that for work to be done by a system, entropy has to increase.

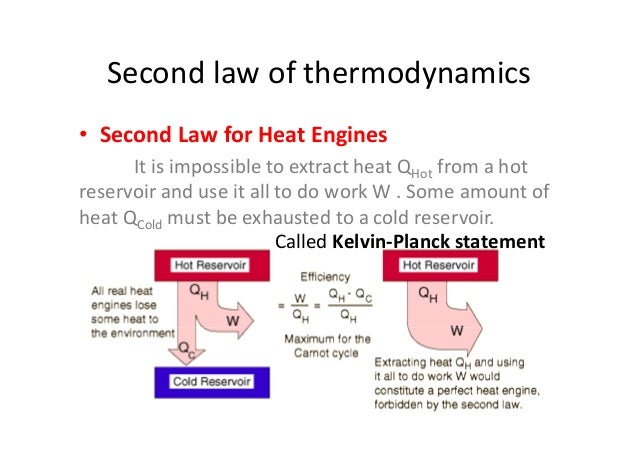
This law explains why heat travels from hotter objects to colder objects. The second law of thermodynamics states that the entropy (spontaneity) of an isolated system will increase over time. The relationship between energy, heat, and work is represented mathematically with the equation: ΔU = w + q, where the change in internal energy of the system is represented by ΔU. The first law of thermodynamics is related to the law of conservation of energy and explains that energy can be exchanged between a system and its surroundings as either heat flow or work. The zeroth law of thermodynamics explains that if two separate isolated systems are both in equilibrium with a third isolated system, then the first two systems are also in equilibrium with each other. These laws describe the energy-related interactions of physical systems with their surroundings. As described by the science of thermodynamics, a set of four laws govern the relationship of heat and temperature to energy and work.


 0 kommentar(er)
0 kommentar(er)
